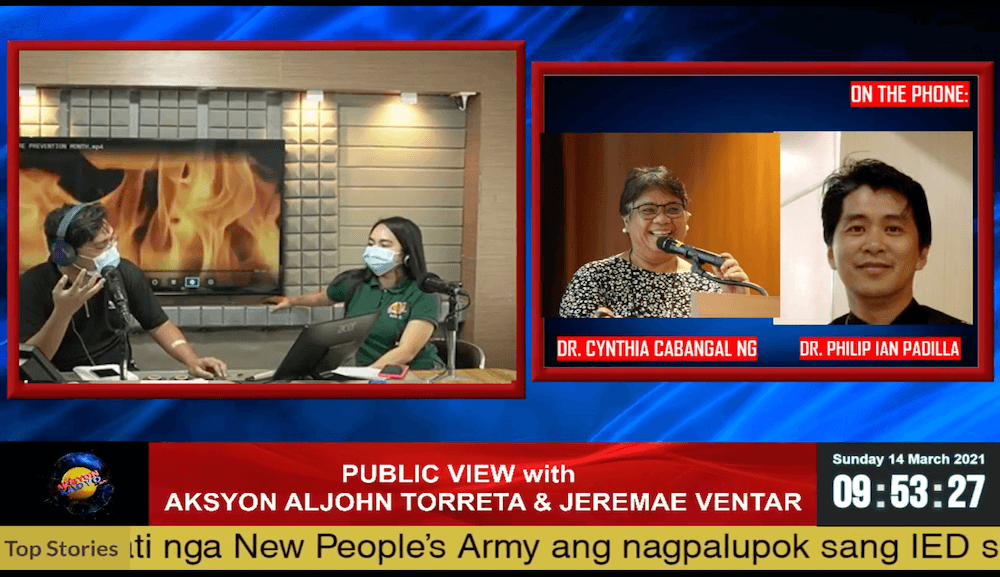
In an interview with Aksyon Radyo Iloilo on March 14, 2021, Vice Chancellor for Academic Affairs, Dr. Philip Ian Padilla emphasized that before a vaccine is rolled out for public use, it goes through an expert panel and various agencies.
He also added that with the help of technology, the process of creating a vaccine has been fast-tracked. “It usually takes around five to ten years for a vaccine to be developed but because of the advances of technology, we now have three vaccines that have been approved for emergency use from the Food and Drug Administration (FDA),” Padilla said.
He explained that the vaccines go through an expert panel convened by the Inter-Agency Task Force (IATF) then it has to be approved by the Food Drug Administration (FDA), the Department of Health (DOH), the Health Technology Assessment Council (HTAC), National Immunization Technical Advisory Group (NITAG), and the National Adverse Events Following Immunization Committee (NAEFIC).
Padilla added that the FDA bases their decisions on reliable and scientific data before approval. He also said that the adverse effects of vaccines are minimal. Acquiring COVID-19 is more life threatening.