In response to the many apprehensions about getting vaccinated for the COVID-19 virus, the UPV Health Services Unit (HSU) partnered with the UPV Healthy Lifestyle and Wellness Committee for a webinar entitled “COVID-19 Vaccines Literacy” on April 15, 2021.
Dr. John Lester Sartaguda of the UPV HSU was a most capable resource person for the topic. He has participated as a sub-investigator in more than 15 clinical trials in different fields, the most recent of which involves COVID-19 at the West Visayas State University Medical Center.
The lecture was most comprehensive and highly informative in scope, starting with how COVID-19 was formed and its toll on the human population (with 136, 291,755 confirmed cases including 2,941,128 deaths as of April 14, 2021).
Sartaguda said that projections on how COVID-19 will play out are speculative, but the end game will most likely involve a mix of everything that checked past pandemics. This includes 1.) continued social-control measures to buy time, 2.) new antiviral medications to ease symptoms, and 3.) a vaccine.
He also explained how vaccines work in the most simplified terms possible and why it is important to get vaccinated.
“When someone is vaccinated, they are very likely to be protected against the targeted disease. But not everyone can be vaccinated. People with underlying health conditions that weaken their immune systems (such as those who have cancer or HIV) or who have severe allergies to some vaccine components may not be able to get vaccinated with certain vaccines. These people can still be protected if they live in and amongst others who are vaccinated,” he explained.
“When many people in a community are vaccinated, the pathogen has a hard time circulating because most of the people it encounters are immune. So, the more that others are vaccinated, the less likely people who are unable to be protected by vaccines are at risk of even being exposed to harmful pathogens. This is called herd immunity,” he further elaborated.
He said that this is especially important for those who can’t be vaccinated and may be more susceptible to the diseases we vaccinate against.
“No single vaccine provides 100% protection, and herd immunity does not provide full protection to those who cannot safely be vaccinated. But with herd immunity, these people will have substantial protection, thanks to those around them being vaccinated,” he pointed out.
“Vaccinating protects not only yourself, but it also protects those in the community who are unable to be vaccinated,” he emphasized.
On the issue of the COVID-19 vaccine being called the fastest vaccine ever to be developed, Dr. Sartaguda shared this information:
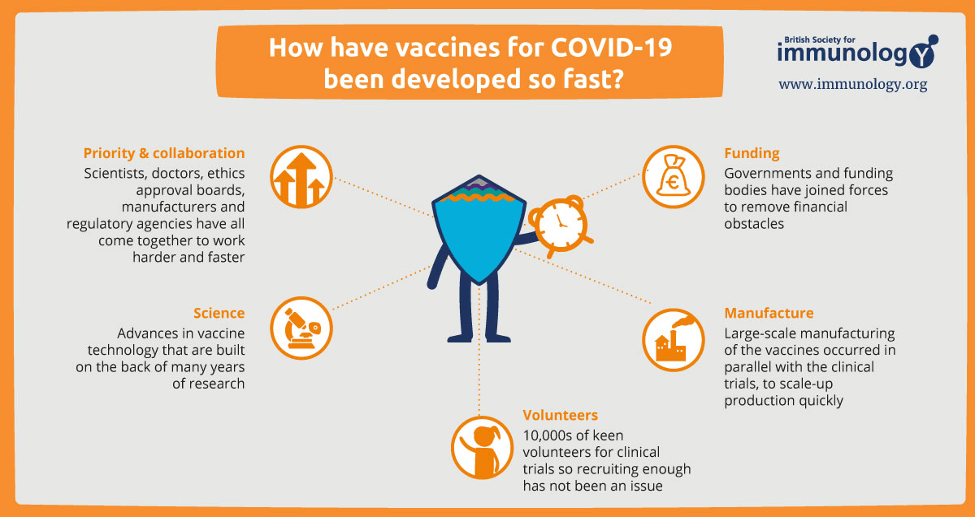
With all these factors working together and once a vaccine passes critical safety and efficacy milestones, Sartaguda said, it will receive emergency use approval (EUA) from the government. In turn, healthcare organizations can start providing the vaccine to patients in a matter of days.
The COVID-19 Vaccine with approved/pending EUA in our Country (Approved for Roll-out) are:
1. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) - January 14, 2021
2. ChAdOx1-S[recombinant] (COVID-19 Vaccine AstraZeneca) - January 28, 2021
3. SARS-CoV-2 Vaccine (Vero Cell), Inactivated [Coronavac] - February 22, 2021
4. Sputnik V Gam-COVID-Vac COVID-19 Vaccine - March 19, 2021
In anticipation of probable questions that may arise from his lecture, Dr. Sartaguda came with FAQ/myths/facts on the COVID-19 vaccine. These are some of them:
Are COVID-19 Vaccines really necessary? Yes, because we continue to be in the midst of a pandemic. So far, “health protocols” have allowed us to cope with the pandemic, such as social distancing, handwashing, and wearing masks, but perhaps the best way to stop this COVID-19 virus is to build enough SARS-CoV-2-specific immunity. No virus has ever eliminated itself by inducing natural immunity (getting a large enough percentage of the population infected). Only herd immunity induced by vaccination was able to eliminate viruses in the past (i.e.smallpox).
Who should NOT be vaccinated? People with contraindications.
• Severe allergic reaction (e.g., anaphylaxis) after a previous dose or to a component of the COVID-19 vaccine
• An immediate allergic reaction of any severity to a previous dose or known (diagnosed) allergy to a component of the vaccine
How about people with other allergies?
• Allergic reactions related to food, pet, venom, or environmental allergies, or allergies to oral medications (including the oral equivalents of injectable medications), are not a contraindication or precaution to COVID-19 vaccination.
• COVID-19 vaccines do not contain eggs or gelatin. People with allergies to these substances do not have a contraindication or precaution to vaccination.
Can a Covid-19 vaccine make me sick with Covid-19? No.
• None of the authorized and recommended COVID-19 vaccines contain a live virus that causes COVID-19. This means that a COVID-19 vaccine cannot make you sick with COVID-19.
• It typically takes a few weeks for the body to build immunity (protection against the virus that causes COVID-19) after vaccination.
• That means it’s possible a person could be infected with the virus that causes COVID-19 just before or just after vaccination and still get sick. This is because the vaccine has not had enough time to provide protection.
After getting a COVID-19 vaccine, will I test positive for COVID-19 on a viral test? No.
• Covid-19 vaccines do not contain LIVE COVID-19 virus
• Antibody Test: May test Positive since your body develops an immune response—the goal of vaccination
If I have already had COVID-19 and recovered, do I still need to get vaccinated with a COVID-19 vaccine? Yes.
• You should be vaccinated regardless of whether you already had COVID-19.
• That’s because experts do not yet know how long you are protected from getting sick again after recovering from COVID-19.
• Even if you have already recovered from COVID-19, it is possible—although rare—that you could be infected with the virus that causes COVID-19 again
• If you were treated for COVID-19 with monoclonal antibodies or convalescent plasma, you should wait 90 days before getting a COVID-19 vaccine.
Can Persons with active Covid-19 infection be vaccinated? No, not right away.
• People who currently have COVID-19 should wait until they have recovered.
○ Recovery of Mild to Moderate COVID-19: 14 days
○ Recovery of Severe to Critical COVID-19: 21days
Can a vaccinated person pose a risk to their unvaccinated household? No.
• The COVID-19 vaccines are not composed of live viruses, so there is no infectious virus to spread from a vaccinated person to others in the home who did not get their shots yet.
• Given that families will not all get vaccinated simultaneously, those who have been vaccinated should continue to practice the same health protocols.
Will a COVID-19 vaccine alter my DNA? No.
• COVID-19 vaccines do not change or interact with your DNA in any way.
• mRNA from a COVID-19 vaccine never enters the nucleus of the cell, which is where our DNA is kept; thus, it cannot affect or interact with our DNA in any way
• Viral vector vaccines use a modified version of a different, harmless virus (the vector) to deliver important instructions to our cells to start building protection. The instructions are given in the form of genetic material. This material does not integrate into a person’s DNA. These instructions tell the cell to produce a harmless piece of virus that causes COVID-19.
Vaccine Considerations for People with Underlying Medical Conditions
• People who have weakened immune systems
• People who have autoimmune conditions
• People who have previously had Guillain-Barre syndrome (GBS)
• People who have previously had Bell’s palsy
On Pregnancy and Breastfeeding
• Pregnant individuals should be free to make their own decision regarding COVID-19 vaccination
• They should be encouraged to discuss vaccination considerations with their clinical care team when feasible. Documentation of such a discussion should not be required prior to receiving a COVID-19 vaccine
• Further, pregnant individuals should not be denied COVID-19 vaccine(s) because of their pregnancy-status alone
• Based on how these vaccines work in the body, experts believe they are unlikely to pose a specific risk for people who are pregnant. However, there are currently limited data on the safety of COVID-19 vaccines in pregnant people
In addition, the Philippine Obstetrical and Gynecological Society issued this statement on January 29, 2021:
• The decision to receive the COVID-19 vaccine is an individual informed decision.
• There are no studies at present on the safety of administration of COVID-19 vaccine among pregnant and breastfeeding women.
• The mRNA COVID-19 vaccines should be offered only to pregnant and breastfeeding women classified to be in the high-risk group recommended for priority vaccination.
• Pregnant and breastfeeding women with comorbidities that add to their risk of severe disease may be vaccinated in consultation with their health care provider.
• For women administered with the Pfizer BioNTech COVID-19 vaccine, the World Health Organization (WHO) does not recommend discontinuing breastfeeding after vaccination
• Immunization should be avoided in most patients during the first trimester to avoid a coincidental association with spontaneous abortion, which is common in the first trimester
• A pregnancy test is not a requirement prior to COVID-19 vaccination
Is it safe for me to get a COVID-19 vaccine if I would like to have a baby one day? Yes.
• If you are trying to become pregnant now or want to get pregnant in the future, you may receive a COVID-19 vaccine when one is available to you.
• There is currently no evidence that the COVID-19 vaccination causes any problems with pregnancy, including the development of the placenta.
• There is no evidence that fertility problems are a side effect of any vaccine, including COVID-19 vaccines.
Can we mix and match the Covid-19 vaccine? No. Vaccine brands are not interchangeable at this time.
WHO recommends that the same product should be used for both doses.
If different COVID-19 vaccines are inadvertently administered in the 2 doses, no additional doses of either vaccine are recommended at this time.
US CDC gives a stronger statement: mRNA COVID-19 vaccines are not interchangeable with each other or with other COVID-19 vaccine products. The safety and efficacy of a mixed-product series have not been evaluated.
Both doses of the series should be completed with the same product.
UK National Health Service is conducting research to discover the effectiveness of combining two different vaccines – COM-COV study.
Dr. Sartaguda said that what is known and still being learned are:
• COVID-19 vaccines effectively prevent COVID-19 disease, especially severe illness and death.
• Other prevention steps help stop the spread of COVID-19. These steps are still important, even as vaccines are being distributed.
• how effective the vaccines are against variants of the virus that causes COVID-19. (Early data show the vaccines may work against some variants but could be less effective against others.)
• how well COVID-19 vaccines keep people from spreading the disease.
• how long COVID-19 vaccines can protect people.
He said that instead of asking what is the best vaccine for me, we should ask: Which one will keep me alive? Which one will keep me out of the hospital? Which one will end the pandemic? To which he answered: Any of them.
“So the best vaccine right now is the one being offered to us. You should get any COVID-19 vaccine that is available when you are eligible. Do not wait for a specific brand. All currently authorized and recommended COVID-19 vaccines are safe and effective. CDC does not recommend one vaccine over another,” he said in conclusion.
Two doctors from the UPV HSU served as panel reactors. The first was Dr. Paulene C. Azucena, an alumna of the BS Biology program of UPV, and earned her Doctor of Medicine from the WVSU College of Medicine. She took her internal medicine residency at St. Paul's Hospital, a diplomate and fellow of the Philippine College of Physicians, presently practices as a private internist, and is a Medical Officer III at the UPV HSU.
The other was Dr. Joy N. Javier, a graduate of the BS Biological Sciences at the West Visayas State University, earned her Doctor of Medicine at the Emilio Aguinaldo College of Medicine. She is a member of the Philippine College of Lifestyle Medicine. Presently Medical Officer III at the San Joaquin Mother and Child Community Hospital and reliever medical officer at UPV HSU.
Both doctors shared their experience of having been vaccinated. They commonly shared experiencing the expected mild pain at the injected area. Dr. Azucena experienced headaches, but both doctors said that they experience no adverse effects on the general aspect.
Dr. John Lester Sartaguda is also an alumnus of the BS Biology program of UP Visayas, who earned his Doctor of Medicine at the West Visayas State University College of Medicine. He did his internal medicine residency training at WVSUMC Department of Medicine; and is a diplomate and fellow of the Philippine College of Physicians. He is presently a Medical Officer III at the UPV HSU.
Dr. Riza Subade of the UPV HSU introduced the three doctors and facilitated the Q & A. UPV HSU Chief, Dr. Cecilia Villaruz, welcomed the 84 online participants.
UPV Healthy Lifestyle and Wellness Committee Chair Mary Lyncen Fernandez expressed her appreciation for the HSU partnership, particularly the support of the doctors and the extensive lecture of Dr. Sartaguda. She likewise thanked the UPV WellCom members for putting together the event. These members are Dr. Riza O. Subade, Lenilyn B. Gallos, Rose F. Flame, Nica M. Molo, Marites E. Geonanga, Herbert Nalagay, Maureen Kay C. Ongo, Prof. Catherine B. Anecita, and Prof. Rhoma Grace V. Pandan.